The Salty Science of the Aluminum-Air Battery
by Dr. Stephanie Chasteen, Dr. Dennis Chasteen, and Dr. Paul Doherty
Online Supplementary Material
Index:
The construction of this cell is borrowed largely from the Exploratorium’s Square Wheels book.
1. Scissors
2. Lamp cord, Christmas tree lights, or other copper wire
3. Wire strippers
4. Aluminum foil (about 20 cm)
5. Table salt
6. Pitcher of water
7. 4 plastic cups
8. 5 alligator clip leads
9. Light emitting diode (LED) or a 12V DC mini buzzer (try RadioShack
#273-065 or #273-075)
10. Multimeter
11. Optional: vinegar or bleach
 Mix
about 20 g of salt with 400 ml of warm water. The amounts are not that
important, but this will give you a solution that is about 5% salt by
weight.
Mix
about 20 g of salt with 400 ml of warm water. The amounts are not that
important, but this will give you a solution that is about 5% salt by
weight.
Cut the aluminum foil into sections about 10 cm x 10 cm. Fold each in
half, and then in half again lengthwise, to end up with a folded “paddle”
about 2.5 cm x 10 cm (photo, left).
Cut the copper wire into 5 sections about 10 cm long. Strip the insulation off one half of the wire, and off about 2.5 cm of the other end of the wire. This leaves a short section of insulation in the middle of the wire, holding it together. Separate the strands of the stripped half of wire, so it looks like a broom (photo, right).
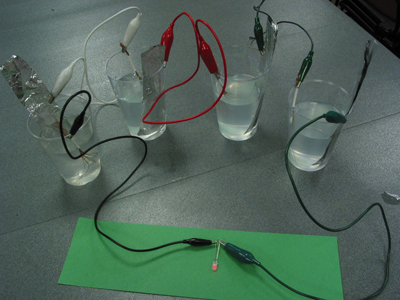
Using the alligator clip leads, clip the aluminum foil to one side of
a cup. Clip the wire strands of the "broom handle" part of the
copper wire to the other side of the cup, so the spread "broom"
section is inside the cup. This is now one “cell” in your
battery. Connect the remaining 3 cups in a similar way, with the alligator
clip lead attached to the copper electrode of one cup connecting to the
aluminum electrode of the next cup. Make sure the copper and aluminum
is not touching within each cup. See picture, left.
Connect the final two leads to a multimeter, LED, or piezoelectric buzzer. Fill the cups with the saltwater solution. What happens?
B. The Role of Vinegar in the Cell
In the TPT paper, we stated:
Actually, a cell made entirely of vinegar water (acetic acid) will work just fine, since the H+ and acetate- ions from the dissociation of the weak acid make the solution conductive. A squirt of vinegar to your saltwater cell will make the LED glow brighter. But don’t be fooled – stirring the solution does the same thing. Upon settling, the performance of the vinegar/salt cell is generally comparable to that of the saltwater cell alone.
However, you’ll probably find that vinegar/salt cells maintain their current for a longer time than saltwater cells. If you leave a vinegar/salt cell overnight, the surface of the copper will not be coated with a reddish oxide, but stays shiny and clean. The acetate in vinegar tends to dissolve the cuprous oxide coating as it forms, by forming a complex with the Cu(II). This allows better contact with the solution and thus better electron transfer over time.
Adding vinegar to the saltwater battery also changes the cell chemistry.
Adding vinegar will result in a pH of about 2-3. Equations 1 and 2 are
no longer favored at low pH, and under acidic conditions Equation 3 (the
overall reaction) becomes
(5) 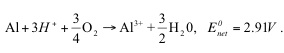
Thus, when vinegar is added to the cell, little or no white aluminum hydroxide
(Al(OH)2(S)) is seen. You should also see more hydrogen
bubbles when operating at a lower pH, since the equation in endnote (11)
is more favored:![]()
The copper also turns quite red in a vinegar cell, indicating the oxidation
of copper to form cuprous oxide. This is because the copper ions, Cu2+,
may also react with the aluminum metal to form a red cuprous oxide (Cu2O)
coating on the aluminum. The aluminum may also turn somewhat dull -- this
is not a coating, as aluminum hydroxide is not formed in this vinegar
cell. Rather, as the aluminum metal corrodes, microscopic pitting dulls
the formerly smooth finish.
C. The Role of Bleach in the Cell
In the TPT paper, we stated:
You will find you’ll get a much more stable and powerful cell if you add a teaspoon of bleach to the saltwater (or to plain water) with current and voltage around 10 mA and 1 V, respectively with only the multimeter in the circuit. So a bleach-powered cell produces about 5 times as much power! Why is that?
When bleach is added, the battery is no longer an air battery; instead of oxygen from the air, sodium hypochorite (NaOCl), the major constitutent in bleach, and hypochorous acid (HOCl), a minor constituent are reduced. The full equations for this cell can be found online. This cell potential (3.93 V) is quite a bit higher than the 3.12 V for the saltwater battery (Equation 3). So this reaction proceeds more rapidly, generating more electrons per unit time and thus greater current. We also observed a higher voltage. Another indication that the reaction proceeds more rapidly for the bleach battery is the striking abundance of white fluffy particulate as the cell is left over time – this is Al(OH)3(S).
Here is the detailed chemistry behind that statement:
In the bleach battery, sodium hypochorite (NaOCl), the major constitutent
in bleach, and hypochorous acid (HOCl), a minor constituent, are reduced,
according to equations 6 and 7:
(6) ![]()
(7) ![]()
The possible reactions involving aluminum are given by equations 8 and
9:
(8) ![]()
(9) ![]()
Even though there is relatively little HOCl in bleach, the latter reaction
is more favored because of its large potential of 3.93 volts.
Over time, you will see both green cupric hydroxide (Cu(OH)2)
and black cupric oxide (CuO). Black CuO is formed from green Cu(OH)2
by the loss of water, which happens over time.
D. Rathjen and P. Doherty, Square Wheels and Other Easy-to-Build Hands-On Science Activities, Exploratorium, San Francisco, 2002, p.85.
