Heat flows.

Heat flows.

Introduction
Liquid crystal thermometers portray temperatures as colors and can be used to follow temperature changes caused by heat flow. They can be used to observe that heat flows by conduction, convection, and radiation.
Material
Four explorations follow:
How a temperature sensitive liquid crystal
works.
To Do and Notice
Hold a liquid crystal thermometer card over your hand and observe it as it warms up. Notice that it starts out black, then becomes red, yellow, green, blue then black once again. Liquid crystals can be adjusted by the manufacturer to change colors over different temperature ranges, but 30 °C to 35 °C seems to be a common range.
What's Going On
The molecules in the liquid crystal thermometer are called cholesteric liquid crystals. The molecules are rod-shaped. The rods form layers in the liquid crystal, the rods in adjacent layers running in a slightly different direction from those in the layer above or below. From one layer to the next the rods form a structure like the stairs of a spiral staircase. The spiral staircase rises through some distance before the molecules twist around parallel to their original direction. When this distance equals half-a-wavelength of red light, the liquid crystal will reflect red light. When the distance is shorter, matching half a wavelength of blue light, the crystal will reflect blue light. (The wavelength of light is measured in the liquid crystal and is shorter than the wavelength in air.)
This seemed strange to me at first. Why should cold crystals with closely spaced layers reflect red light and warmer more spread out layers reflect blue light? The resolution of this problem came when I found out that the temperature effects not only the spacing of the layers but the twist angle from one layer to the next. When the liquid crystal warms up the layers spread apart slightly, but, in addition, each layer of rods rotates a little more relative to the later below it. This means it takes fewer layers and therefore a shorter distance between layers of parallel rods. So when the temperature increases the spacing between layers in which the rods are parallel decreases. The color goes from red when cold to blue when hot.
The liquid crystals are usually encapsulated, packaged in microscopic plastic spheres. This allows the material to be cut. they are also thermally rugged which allows them to be laminated to protect them from hard handling in a high temperature laminating machine.
To Do and Notice
Change the temperature of the liquid crystal card
by conduction. For example, hold the card against the back of your
hand. As heat flows from your hand to the card the temperature of the
card will increase and it will change color. Sometimes, as the card
changes color the pattern of arteries on the back of your hand
becomes visible. Arteries appear because arterial blood is warmer
than the hand.
Notice that the color of the liquid crystal changes from black to
red, to yellow, green, blue, and finally back to black again.
As a variation on this experiment, hold a quarter between your hands
for a minute, until it becomes warm. Place the quarter on a table
then place a liquid crystal card on the quarter, when it starts to
change color put a second liquid crystal card on top of the first.
Repeat when it changes color. You are observing heat conduction
through the cards.
As a second variation, cut pieces of scrap metal (about 1” x
4”) then glue a piece of the thermal postcard cut to this same
size to the metal. Keep the glue layer thin, spray on adhesives work
well.
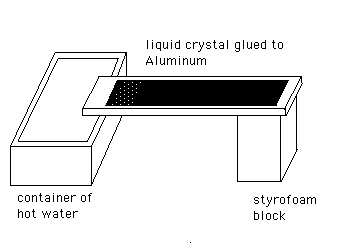
Insert one end of the bar into hot water and observe how the
temperature changes as heat flows by conduction through the rod.
Notice that as the color moves along the rod the distance between the
blue and the red colors broadens. Unlike waves in a string which
propagate without spreading, heat flows by diffusion so that a heat
pulse will spread rapidly as it travels. Place the ends of the bar in
hot water for 15 seconds and then cold water, continue to alternate
between hot and cold water. Notice that the color starts out as
pulses which then spread and blend and never reach the far side of
the card. This is why Morse used electrical signals in his telegraph
and not heat pulses.
Use the stopwatch and ruler time how long it takes the red color to move the first centimeter, the second centimeter, and so on. Notice that the heat pulse takes longer and longer times to travel the same distance. This is another indication that heat conduction is by means of diffusion.
What’s Going on ?
In heat flow by conduction, thermal energy represented by the vibration of molecules moves from one place to another place. The vibrating molecules collide with their neighbors setting them into motion. A high temperature pulse will naturally propagate into a low temperature region as the vibrational energy spreads. If a high temperature pulse which is propagating into a cold metal bar is followed by a low temperature pulse the high temperature pulse will spread both ways. Into the cold temperatures in front of it and behind it. This is why heat flow is different from wave motion. Once a wave (of water, on a string, of sound or light) starts moving in a material it will keep moving regardless of what happens behind it
So What ?
Summer-winter
Heat flows in the ground the same way that it flows in your metal pieces. If you dig down into the ground you will find the most recent temperatures near the surface. Deeper down you will find temperatures at depth representing the average over surface temperatures in the past. The temperature of deeper regions will be an average of past surface temperature. That’s why you can dig down in the New England winter and find unfrozen ground, the average temperature over the year is above freezing. It is also why you can dig down in the arctic in the summer and find permafrost.
To do and notice
Make a cup of hot water. Hold liquid crystal card
in a vertical plane about a centimeter over the hot water (or a
glowing lightbulb) and watch the temperatures change. Notice the
shape of the colored region, it resembles a plume or a candle
flame.
Hold an ice cube about a centimeter over the card. Notice the color
changes brought on by the downward flowing cold air.
What’s going on?
Air near the liquid is warmed by conduction, the warmer air rises transporting thermal energy to the card. Air expands when it is warmed becoming less dense than the surrounding air and therefore buoyant. Similarly, air falls when it is cooled. Convection occurs when thermal energy is transported from one place to another by the motion of a liquid or a gas. We can break convection into two types: natural convection in which the motion is driven by differences in density of fluids, and forced convection in which the fluid is moved by pumps or fans.
So What?
During a hot summer afternoon the warm ground heats the air, the air then rises forming large convective plumes called thermals. Soaring birds and glider pilots seek out thermals and enter them to be lifted to higher altitudes by the rising air. Convection also occurs within the tall towers of cumulus clouds.
Forced convection is used in convection ovens where fans circulate the hot air around the food.
To Do and Notice
Move the card from shadow into sunlight. Notice how it changes color. (You can slow down the time it takes the card to change color by gluing it to another material such as a sheet of metal or plastic.)
Repeat the experiment but cast the shadow of a finger onto the card.
Repeat the experiment but hold the card at an angle, 45°, to the sun. Notice that the card takes longer to change colors than a card held perpendicular to the sunlight.
Hold the card a couple of centimeter from the side of a lamp so that light from the lamp hits the card, notice the color changes. (Don’t hold the card over the lamp or the card will be warmed by both convection and radiation.)
You can measure the power of the sun using a thermal postcard and the inverse square law. (see the Solar Thermal activity.)
What’s going on?
Energy is being carried from the sun to the card by radiation. Visible light, infrared and ultraviolet radiation carry energy from the sun through the vacuum of space.
Your shadow blocks the radiation just as the shadow of the moon blocks the sun’s radiation during an eclipse.
When you tip the card at an angle a smaller amount of solar radiation is spread over the card, so less energy is spread over the card and it takes longer to change its temperature.
You can wrap the thermal card around an empty plastic bottle, e.g. a 2 Liter bottle. Place the bottle in the sun with the middle of the card pointing at the sun. Notice how the place on the card pointing toward the sun changes temperature most rapidly and becomes warmest, while the parts wrapped around the sides of the card change temperature more slowly. This models why the earth's srface is warmest on the summer days and cooler in the winter.
So What?
The angle the sunlight makes with the surface of
the ground changes during the year, this is why we have seasons. When
the sun is more overhead in the summer, sunlight strikes the ground
in the same way it strikes a thermal card held perpendicular to the
sunlight. The card warms up quickly and so does the ground. When the
noonday sun is low in the sky as it is in the winter sunlight hits
the ground at an angle and warms it up more slowly.
Evaporation
To Do and Notice
Try to show temperature changes due to evaporation.
This is a good challenge, try it.
Evaporation is a cooling process to show it first heat the card until it is blue or warmer, then sprinkle some water on the card and blow on the water. The evaporating water will cool the card.
What's Going On?
It takes energy to free a water molecule from liquid water. In a liquid, water molecules were in a low energy state because they were strongly attracted to its neighbors. When water evaporates, energy is removed from the liquid water. In addition, when water does evaporate the fastest molecules leave first. The molecules left behind are slower. For both of these reasons evaporation is a cooling process.
So What?
Humans use evaporation of water, sweat, to cool their bodies. The evaporation of a small amount of water produces a large cooling effect.
|
Scientific Explorations with Paul Doherty |
4 Nov 99 |