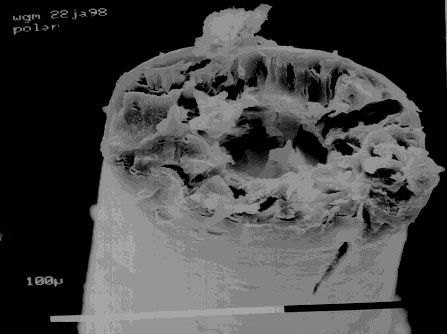
An electron microscope image of a cross section of polar
bear fur. Image copyright 1998 by Bill May. Used by
permission.
Popular Errors
Often scientific discoveries make the news and spread across the world, then are later found to be false. The news of the correction often doesn't spread as widely as the original report. Here are a few classic cases where incorrect science is widely known.
There are some good collections of sites providing information on popular errors in science.
The Urban Legends of Science Page at http://www.urbanlegends.com/faq2k/science_index.html
Alistair
Fraser Bade Science Page at
http://www.ems.psu.edu/~fraser/BadScience.html
He specializes in meteorology, Astronomy and Chemistry.

An electron microscope image of a cross section of polar
bear fur. Image copyright 1998 by Bill May. Used by
permission.
The original researchers noted that while polar bears were white in the infrared and the visible, they were black in the ultraviolet. These observations were correct. However they speculated that the reason they were black in the UV was that their fur was a fiber optic and transported the UV radiation to and from their black skin. Later research showed that this was incorrect, the polar bear fur was black in the ultraviolet because it is made from keratin which strongly absorbs UV.
I actually got a bit of polar bear fur from the San Francisco Zoo and sent it to my friend Bill May who has an electron microscope. When he looked at the fur he found that it was a hollow shaft. This is not the way to make a fiber optic. To make a fiber optic the center of the shaft should have a lower velocity of light than the outside, a shaft full of air has a higher speed inside and will disperse the light. In addition we found that the hollow cylinder of the hair shaft is full of small bumps. These scatter the light and make the fur white even when the outside of the hair is wet.
A full Page of polar bear fur optical property information.
The original report noted that glass seemed to be thicker at the bottom of ancient windows. It then went on to speculate that the thickening was caused by the flow of the glass under gravity.
Later research showed that some ancient windows were thicker on top or on the sides. So the original observations were incorrect.
The Corning Museum of Glass website contains a great resource of information on glass. http://www.cmog.org/page.cfm?page=77
It notes that room temperature glass has a viscosity of 1022 poise. The viscosity of a liquid controls how fast it flows under gravity. (SAE 30 motor oil has a viscosity of about 1 poise, water is 0.01 poise.) The viscosity of glass is so high that you could wait the entire age of the universe and see no measurable thickening of the glass under earth gravity.
Solid, liquid, gas at the earth's surface
One simple set of definitions of a solid, liquid and gas is that under earth gravity:
A solid keeps its shape.
A liquid fills a container, assuming the shape of the container. (And, in addition, has a discontinuity in density at the surface of the liquid, the discontinuity in density allows us to discriminate between a liquid and a dense gas like sulfur hexafluoride which fills the container but which has a gradual density change at the interface with air.)
A gas in an open container expands to fill the room.
Solid, liquid, gas in freefall.
The differences become more obvious in a spacecraft in freefall where no container is needed. The solid maintains its shape. The liquid is pulled into a sphere by surface tension. And the gas expands to fill the spacecraft.
The Engineering definition for solid liquid or gas.
The ASTM (American Society for Testing and Materials) has a standard for determining whether something is a liquid. ASTM (1996) "D4359-90: Standard Test Method for Determining Whether a Material Is a Liquid or a Solid."
Here is their method: The material under test is held at 100deg.F (38deg.C) in a tightly closed can. The lid is removed and the can inverted. The flow of the material from the can is observed to determine whether it is a solid or a liquid.
They specify that the material in a 1 Liter container must flow over 2 inches in three minutes to be considered a liquid.
Under this test glass is definitely a solid.
The Physicists definition of a liquid.
A liquid will flow by shear, the displacement of one layer of atoms with respect to the neighboring layer, cold glass does not flow by shear and so is not a liquid by the physicists definition. A solid bends under load then resumes its original shape when the load is removed.
Further reading
On antique windowpanes, http://dwb.unl.edu/Teacher/NSF/C01/C01Links/www.ualberta.ca/~bderksen/windowpane.html
Popular Error: Airplanes fly because of either Bernoulli
velocity/pressure relation or Newton's action
reaction.
A great aerodynamics site by Tom Benson a longtime NASA aerodynamics researcher who can write clear explanations is
The Beginners Guide to Aerodynamics at
http://www.grc.nasa.gov/WWW/K-12/airplane/short.html
He does the best job I have ever seen of explaining the complexities of aerodynamics using correct physics.
He says
The real details of how an object generates lift are very complex and do not lend themselves to simplification. For a gas, we have to simultaneously conserve the mass, momentum, and energy in the flow. Newton's laws of motion are statements concerning the conservation of momentum. Bernoulli's equation is derived by considering conservation of energy. So both of these equations are satisfied in the generation of lift; both are correct.The simultaneous conservation of mass, momentum, and energy of a fluid (while neglecting the effects of air viscosity) is called the Euler Equations after Bernoulli's student, Leonard Euler. If we include the effects of viscosity, we have the Navier-Stokes Equations
This is also what I say.
The Bernoulli equation expresses the conservation of energy and if it is used correctly can explain lift.
The Newton equation expresses the conservation of momentum, to deflect the motion of flowing air downward, a force must be exerted on the air. The reaction force is exerted upward on the wing. This equation also may be used to completely explain lift.
But both techniques are often applied incorrectly particularly by opponents of each theory.
For more detail see my Aerodynamic Thoughts
Here are some common problems.
Popular Error: Air flow over the top of the wing must meet up with airflow under the bottom.
Some people say that airflow over the top of the wing must be faster than airflow under the wing because a parcel of air that splits at the front of the wing must meet up again at the rear, (It doesn't). The faster airflow results in lower pressure on top of the wing and lift. In reality experiments show that airflow over the wing is much faster than the airflow speed needed to have the parcel of air rejoin at the rear of the wing. The airflow over the wing is faster to satisfy the law of conservation of mass flow.
Here is a photo:

Bernoulli's equation is
P1 + 1/2pv12 + pgh1 = P2 +1/2pv22 + pgh2
Where you must choose two points connected by a streamline of airflow, points 1 and 2.
P1 is the pressure at point 1.
v1 is the velocity of airflow at point 1.
h1 is the height of the fluid at point 1.
and similarly for point 2.
p is the density of air which is assumed to be a constant. (Air is assumed incompressible)
g is the acceleration of gravity 9.8 m/s2
This is the law of conservation of energy.
Each side contains the energy per unit volume of fluid.
Note, the relative velocity of the fluid with respect to a surface has nothing to do with Bernoulli's law it is the velocity change from one point on a streamline to another.
For more information see:
Bernoulli's
equation on the hyperphysics site at
http://hyperphysics.phy-astr.gsu.edu/hbase/pber.html
Other aerodynamic explorations.
Airflow over and under a paper strip. Cut a strip of paper 3 inches wide and 11 inches long. Hold it in front of your lips and blow over it. The high speed airflow over the strip will reduce the pressure above the strip allowing atmospheric pressure below the strip to lift it to nearly horizontal.
Blowing between cans to make them move together or apart.
The force on a curving
ball.
Popular Error: A candle in a jar burns oxygen so that when the
candle goes out water is drawn up inside the jar.
Put a candle in a tray of water. Light the candle and cover it with a jar that is at least 3 times as high as the candle. Notice that the candle goes out and water rises into the jar.
Many texts attribute the water rise to the burning of the oxygen by the candle. They say the candle uses up the 21% of the air that is oxygen and then goes out. The water then rises to fill the lost 21% of the volume that has been removed. This is incorrect.
First of all the candle doesn't burn up much of the oxygen, a mouse can still live happily in the jar even when a flame has burned out.
I have experience with this myself. I played the role of the mouse. I was cooking dinner inside a tent that had been sealed off from atmospheric oxygen by heavy wet snowfall. In the middle of cooking the stove went out. I tried to relight the stove but matches would not burn. I felt no problem breathing. It occurred to me that the matches were not burning because there was not enough oxygen. I opened the door to the tent and the matches and stove burned again.
Second. When wax burns it produces more combustion products than the amount of oxygen it consumes. A typical reaction might be:
C25H52 +38 O2 = 25 CO2 + 26 H2O
38 molecules of oxygen are consumed and 51 molecules of combustion products are created.
A complication to this line of thought is that the water gas created by combustion dissolves into the available water or condenses into droplets of water that line the inner surface of the glass container. When the water is removed from the gas state to the liquid state it no longer contributes to gas pressure. The carbon dioxide is soluble in water and will slowly dissolve into the water. Thus the water should still rise due to the combustion process.
However, the main reason that the water rises is due to the cooling of the hot air around the candle, the cooler air contracts lowering the pressure inside, full atmospheric pressure outside the jar then pushes water into the jar.
To separate the affects of cooling and gas dissolution. Repeat the experiment using cooking oil instead of water. Carbon dioxide is not very soluble in complex oils.
You can also hold the jar over the candle but keep the bottom of the jar above the surface of the water until the candle goes out then quickly lower the jar into the water.
The jar is at atmospheric pressure until the candle goes out When the gas cools the water rises into the jar.
At the workshop we placed an unlit candle inside a jar in a tray of water. Then we inserted a piece of tygon tubing under the water and into the air space of the jar and used it to suck air out of the jar allowing water to rise up into the jar. We then lit the candle inside the jar using a fresnel lens. A match head was taped to the candle wick to help it light. The heating of the air in the jar rapidly drove out the water then even more gas bubbled out of the jar. When the candle went out water was sucked up into the jar.
Other common Urban Legends of Science
Popular Error: The rubber tires on a car protect you from Lightning.

No The lightning bolt just jumped over a thousand feet through the air to reach your car, it can easily jump through the air beneath your car the final few inches to reach the ground. (This is shown in a photo from a General Electric test in which a manmade bolt of lightning strikes a car.) You are safe inside your car because it is a Faraday Cage. You are surrounded by electrically conducting metal and wet glass. the electric charge transported by the lightning bolt stays on the outside surface of a conductor.
For More information see
http://www.lightningsafety.com
|
Scientific Explorations with Paul Doherty |
|
6 February 2004 |