Charge it

A teacher laughs as the two tapes he holds in his hands repel!
Charge it

A teacher laughs as the two tapes he holds in his hands repel!
Introduction
Use Scotch Magic Tape™ to determine the electric charge of objects.
Material
A roll of 3/4 inch (1.5 cm) wide Scotch Magic Tape™
Optional, scrap wood or smooth cardboard at least 20 cm (9 inches)
square.
Assembly
Pull off two 10 cm (4") lengths of tape and smooth them down onto a table or other firm surface. Call these BASE tapes. Caution, removal of the tapes may mar table surfaces, if you worry about the finish on your tables, put the BASE tapes onto smooth wood or cardboard scraps.
To Do and Notice
0. Two OLD tapes.
Pull off two 10 cm lengths (4”) of tape and hang them from the
edge of a table to age. Let them age. Call these OLD tapes.
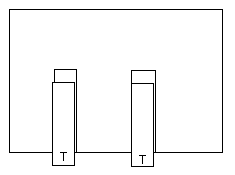
1. Two TOP tapes.
Pull off two 10 cm lengths of tape and stick them down on top of
the two BASE tapes. Fold the end of the tapes back on themselves to
make a handle.
Call these TOP tapes. Optional, mark these with a T.
Quickly pull off the two top tapes and hold one in each hand so
that each hangs down vertically. (If one sticks to your hand shake it
free.) Bring the two tapes near each other and observe what
happens.
They repel.
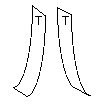
Observation 1. Two tapes treated identically repel.
The sticky side of each tape you removed was pressed against the
smooth side of the BASE tape.
Here is a force that is repulsive, unlike gravity which is always
attractive.
Keep the tapes near each other. Over time notice that they move
closer. The repulsion goes away slowly. On humid days the repulsion
decreases more quickly.
2. Two OLD tapes.
Hold one of the OLD tapes in each hand so that they hang down
vertically. Bring them together. Observe what happens.
Nothing happens, they neither attract nor repel.
(If they repel then let them age longer.)
Observation 2. Two OLD tapes neither attract nor repel.
3. A TOP tape and a BOTTOM tape.
Pull off one 10 cm length of tape and stick it down to one of the
BASE tapes, leave a handle. Call this one a BOTTOM tape and print a B
on one end.
Pull off another 10 cm length and stick it down on top of the BOTTOM
tape. Leave a handle. Call this a TOP tape. Print a T on one end.
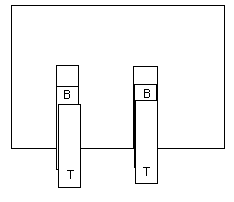
Pull up both TOP and BOTTOM tapes. Keep them together. Stroke them gently between your fingers a few times until they no longer attract your hand.

Pull the tapes apart rapidly and hold one in each hand.
Bring them together. Observe what happens.
They attract.
Observation 3. Two tapes treated oppositely attract.
The sticky side of one was pulled off the smooth side of the
other.
4. A TOP tape and an OLD tape
Stick 10 cm of tape down on a BASE tape. Pull it up to create a TOP
tape. Bring it close to an OLD tape. When the tapes are very close
you may see them attract. (If there is no apparent attraction in this
experiment wait until part 2 where we’ll use more highly charged
materials. Also remember that the charged tapes attracted your
hand.)
Observation 4. Charged tapes attract uncharged tapes.
5. All combinations of TOP and BOTTOM tapes.
Find a partner, pull apart two pairs of TOP and BOTTOM tapes.
Test the following pairs of tapes, and notice:
Observation set 5.
two TOP tapes repel
two BOTTOM tapes repel
Top and Bottom tapes attract
What’s Going On?
The above observations were available to scientists at the end of the 18’th century. Ben Franklin was the first scientist to devise a model to explain the observations. Today we use a model of electric charge derived from Franklin’s model.
The modern model of electric charge says there are two types of electrical charge, particles may posses a positive&emdash; plus&emdash; charge or a negative &emdash; minus &emdash; charge. In normal matter these particles exist in equal numbers so that the net charge is zero. Normal matter&emdash; matter made of atoms&emdash; is uncharged.
Normal matter becomes charged when one material is rubbed against
a different material. Contact between two different materials
transfers electrical charge from one to the other.
The details of the charge transfer are unknown, even today. You might
have read, or been told, that it is the negative charges &emdash;
named electrons &emdash; which move between two objects when they are
rubbed together. As of 1995 scientists do not know the details of
which electric charges actually move when two tapes are pulled apart.
Recent scanning tunneling microscope experiments suggest that entire
atomic or molecular clusters are transferred from one material to the
other. These clusters can transfer either positive or negative
charge.
An editorial aside. This is a good example of how you should always
ask the question “How do you know that?” Keep asking until
someone can point you to an experiment that convinces you of the
truth of their statement.
(An experiment that shows it is electrons that move when tapes are
pulled apart would really make my job of explaining what happens
during electrostatic charging easier.)
Details of the different experiments.
1. When the two TOP tapes were pulled from the BASE tapes they
became charged. Since the two top tapes were treated identically
they have the same charge.
Like charges repel.
We do not know whether the TOP tapes gained or lost, positive or
negative charge.
The excess charge slowly decreased by combining with opposite charges
in the air, or by leaking off the tape.
2. Two old tapes have equal numbers of positive and negative charge, the have no net charge and so, they neither attract nor repel. Actually there are over 1023 positive charges in a gram of tape and an equal number of negatives.
3. When a TOP tape is Pulled from a BOTTOM tape charges move from
one tape to the other. One ends up with an excess of positive charge,
the other with an excess of negative.
Unlike charges attract.
In part 2 we’ll find which one becomes plus and which one
minus.
4. An uncharged OLD tape has many charges on it, over
1023
positive charges and an equal number of negatives. When a
charged object is brought close to the uncharged object, let’s
call it a plus charge for convenience, then the negative charges on
the uncharged tape are attracted by the pluses and move closer while
the positives are repelled and move away. The uncharged tape becomes
electrically polarized. The charged tape has a stronger attraction
for the nearby negatives than its repulsion for the distant
positives.
Thus charged tapes attract uncharged tapes.
5. In order to explain why TOP tapes repel TOP tapes and BOTTOM tapes repel BOTTOM tapes while TOP tapes attract BOTTOM tapes requires the existence of two types of charge called plus and minus.
Ben Franklin’s Model
Ben thought of electric charge as a fluid. After a time, every object, such as the OLD tapes, had a natural level of this fluid.
Objects with their natural level of electrical fluid neither attracted nor repelled each other. (Observation 2.)However, when two objects were rubbed together one object could gain an excess of this electric fluid, he called this object plus. The other would lose fluid, he called this minus. This is the origin of the terms for electrical charge we still use in our modern theory.
Two objects each with an excess of fluid repelled each other.
Two objects with a deficiency of fluid repelled each other.(Observation 1, and 5)
An object with an excess attracted an object with a deficiency. (Observation 3 and 5)
Ben Franklin’s model does not explain the attraction of the charged tape for the uncharged tape.(Observation 4)
Ben’s choice of plus and minus as charge names has lived on after his theory. These are appropriate names because like positive and negative numbers, plus and minus charges when put together in equal amounts have a net charge which is zero.
Ben Franklin’s model was eventually superseded by the modern theory of electric charge. In the modern theory particles exist with positive, negative and zero charges. For example electrons posses negative charge, protons have positive charge, and neutrons have zero net charge.
Stick with me
Introduction
When two materials were rubbed together so that each became
charged, Ben Franklin did not know which one gained charge and which
one lost charge. So he proposed an arbitrary standard. He said, if
you rub amber (solidified tree sap) with cat fur, then, by
definition, the amber becomes minus, while the fur becomes plus.
We still use his proposed standard today.
Rub a comb with a piece of wool and by Ben Franklin’s definition
the comb becomes negatively charged. This definition can be used to
determine the charges on objects.
Material
A roll of Scotch Magic Tape™ 3/4 inch wide (1.5 cm)
a plastic or rubber comb
wool, felt, or hair
Optional scrap wood or smooth cardboard at least 20 cm (9 inches) square.
Optional tape holder material, you can just drape it from a table edge or hold it in a hand.
A film can
enough clay to fill it 1/4 full
two bendable soda strawsAssembly of Optional Tape Holder
Put a lump of clay into a film can.
Press the long straight end of two bendable straws into the clay.
Bend over the shorter ends of each straw until they are horizontal
To do and notice
Pull off two 10 cm (4") lengths of tape and smooth them down onto a table or other firm surface. Call these BASE tapes. Caution, removal of the tapes may mar table surfaces, if you worry about the finish on your tables, put the BASE tapes onto smooth wood or cardboard scraps.
Pull off one 10 cm length of tape and stick it down to one of the
BASE tapes, leave a handle. Call this one a BOTTOM tape and print a B
on one end.
Pull off another 10 cm length and stick it down on top of the BOTTOM
tape. Leave a handle. Call this a TOP tape. Print a T on one end.

Pull up both TOP and BOTTOM tapes. Keep them together. Stroke them gently between your fingers a few times until they no longer attract your hand.

Pull the tapes apart rapidly and hold one in each hand.
Hang the two tapes from the horizontal arms of the straws. From
our previous experiment we know that one of these is positive the
other is negative.
If you are using your own comb and your hair is clean and free of oils or additives comb it, otherwise rub the comb with wool. The comb is now negative by definition.
Slowly bring the comb from far away toward the B tape.
Notice that the comb repels this tape.
Now bring it toward the T tape. The comb attracts this tape.
(Watch out! When the comb gets close to either tape it will attract
the tape. So you should always observe whether the comb attracts or
repels at a distance, not close-up.)
Since like charges repel, the B tape must have the same negative
charge as the comb.
You can now use your hanging tapes to find the charge on an
object.
After the tapes have hung for a time they will lose their charge
becoming OLD tapes.
Bring the comb up to a 10 cm long hanging piece of old tape.
Notice that the comb attracts the old uncharged tape.
The old tape is not charged and yet the charged comb attracts it.
Observation 4: Charged objects attract uncharged objects.
(Well all I can really say at this point is that negatively charged
objects attract uncharged objects.)
Use your tape electroscope to measure the charges on objects. Try rubbing balloons with hair, rub combs with plastic bread bags, rub acrylic with silk, rub Styrofoam with wool, and rub PVC water pipes with paper.
Remember that only repulsion can be used to reliably determine the
charge on an object.
Attraction can result from opposite charges or when one object is
charged and the other uncharged.
Objects repelled by the T tape are positive, those repelled by the B
tape are negative.
The closer the comb is brought to the tape the further the tape moves. This shows that the force between the objects gets stronger as the objects get closer. When the comb approaches the tape very closely, the attraction from polarization can overwhelm the repulsion from the like charges. So watch what happens when the tapes are far from an object.
What’s Going On?
Electric charge produced by rubbing two objects together is known
as triboelectricity.
The tape electroscope can be used to determine the charge on
objects.
Objects charged by rubbing can be arranged in order of which becomes
become positive and which become negative when they are rubbed
together. This ordered list called the triboelectric series.
In general, the further apart two objects are in the triboelectric
series the larger the charge they acquire when rubbed together.
There are several different versions of the triboelectric series,
different versions of the series have different ordering of nearby
materials, they all agree on distant materials. Here is a portion of
the triboelectric series from the book “Natures
Electricity.1” by Charles K.
Adams, TAB books, 1987
Positive
Air
Human flesh
rabbit fur
glass
Human hair
nylon
wool
cotton
amber
hard rubber
rayon
polystyrene foam (Styrofoam)
polyethylene
Teflon
Negative
This series predicts that a rubber comb becomes negative when rubbed with wool and positive when rubbed with a polyethylene bread bag.
Scientists do not know what charges are transferred when two materials are brought together and then separated. Images created with scanning tunneling microscopes shows that entire clusters of atoms are transferred when a tungsten electrode touches a gold surface. Contact can thus transfer positively charged clusters of atoms or negatively charged clusters. This means that rubber can become negatively charged when it is rubbed with wool either by acquiring negative ion clusters from the wool or by giving positive ion clusters to the wool. At the moment we cannot tell which is happening.
Why the tapes lose charge on Humid days
The two tapes on your electroscope will lose their charge slowly,
over many minutes on dry days, and more rapidly, in a minute or two,
on humid days.
On humid days there are many so called large ions in the air as well
as uncharged small water drops. A charged object will attract
opposite charged ions from the air. These will slowly cancel the
charge on the object. Small uncharged water drops will also be
attracted. These will add a conducting coating to an object which
will allow the charge to be conducted off the object.
etc. 1
Atoms are made of positively charged protons, negatively charged electrons and uncharged neutrons. Protons and electrons have equal and opposite charges. Neutral atoms have an equal number of protons and electrons. It is possible for atoms to gain or lose electrons and to become charged either plus or minus. Charged atoms are called ions.
etc. 2
One fundamental property of a particle of matter is its electric
charge, another is its mass.
The mass determines the gravitational attraction. There is only one
type of mass and it leads to gravitational attraction.
There are two types of electric charge. A particle may also be
uncharged.
We call the two types of charge plus and minus. Combining an equal
amount of plus charge to minus charge leads to an object with no net
charge.
Particles called electrons have one unit of negative charge.
Protons have one unit of positive charge.
Neutrons have no charge.
Photons have no charge.
Etc 3
Actually, uncharged atoms and molecules weakly attract each other, the electrons move around so that the atoms are sometimes plus on one side and minus on the other. These polarized atoms weakly attract other similarly polarized atoms. The resulting force is called the Van der Waals force, it holds helium atoms together to form liquid helium when the temperature is cold enough, 3 K.
|
Scientific Explorations with Paul Doherty |
|
30 May 2000 |