CSET Thermodynamics
The zeroth law of thermodynamics: There is a thing called temperature.
Note centigrade was discontinued in 1948, scientific temperature is now measured in degrees Celcius, °C. or in kelvins, K. (Note the unit name "degree Celcius" is the only unit named after a person that contains a capital letter, also the word degree does not precede the word kelvin. http://en.wikipedia.org/wiki/Celsius
Temperature
The 1890's definition of temperature based on the ideal gas laws and written in modern language is that for an ideal gas:
The temperature of an ideal gas is the average random Kinetic Energy of translational motion per molecule.
Every one of these words is important:
Average, the gas molecules have a wide
range of different speeds, we need to take an average. (The
distribution of speeds will be a Maxwell Boltzman distribution.)
A subtle point about average is that it is required that the average
be over more than one molecule, so temperature is not defined for a
single molecule.
Random, the center of mass motion of a bunch of molecules is removed from the definition of temperature, only the motions relative to the center of mass motion matter. This means that the temperature of a gas remains the same in inertial reference frames, those that are moving at a constant velocity or at rest with respect to you.
Kinetic energy, 1/2 m v^2,
translational, only the center of mass translational motion of each molecules counts toward temperature, rotational and vibrational energy do not count.
per molecule, temperature is defined per molecule in an ideal gas. Thus if you cut a box of gas molecules in half the temperature remains the same, even though the total energy contained in the system is cut in half.
Ideal gas, this definition only applies to an ideal gas, it does not apply to liquids, solids, or non-ideal gasses. This is OK because you can find the temperature of a solid or liquid by putting it into contact with an ideal gas, the temperatures of the two systems will become the same and then you can use the ideal gas definition.
Temperature measured in kelvins is proportional to the average random Kinetic Energy of translational motion per molecule. The constant of proportionality is 1/(3/2k), where k is boltzman's constant 1.38 x 10^-23 J/K.
(1/2mv^2)average per molecule = 3/2 kT
Phases
Solid: in the absence of gravity a solid will hold a non-spherical shape.
Liquid: in the absence of gravity a liquid will pull itself into a spherical shape,
Gas: in the absence of gravity a gas will expand to fill a container assuming the shape of the container.
Fluid: a gas or liquid, a fluid will shear under stress and remain sheared when the stress is removed.
and sometimes
plasma: a gas in which all atoms have been ionized
Note that room temperature glass is a solid.
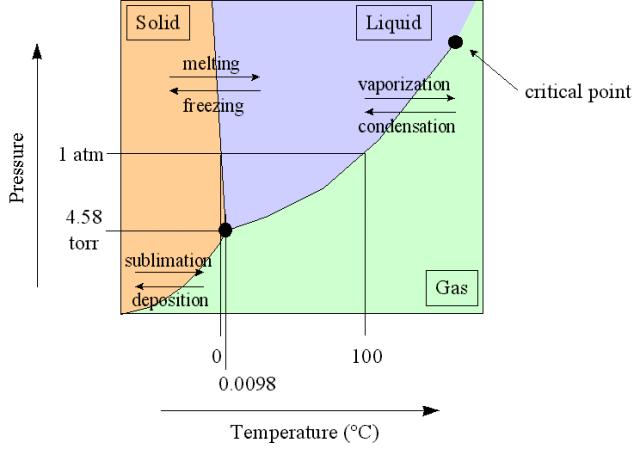
Phase Diagram
A plot showing phase versus temperature and pressure

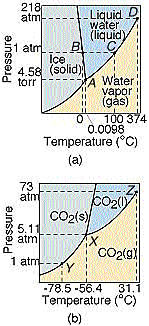
And here is the phase diagram for carbon dioxide compared to water.
Here is a good site with notes on the phase diagram http://wine1.sb.fsu.edu/chm1045/notes/Forces/Phase/Forces06.htm
And another good site with a good set of questions
http://www.science.uwaterloo.ca/~cchieh/cact/c123/phasesdgm.html
To calculate the energy, U, needed to melt ice use the latent heat of
fusion, Lf.
Or the energy, U, needed to vaporize water, the latent heat of vaporization, Lv.
U = m Lf with Lf = 335 J/g for water
U = m Lv with Lv = 2272 J/g for water
example to vaporize a kilogram of water it requires
U = m Lv = 1000 g * 2272 J/g = 2.272 x 10^6 J
To convert to calories use 1 c = 4.184 J
So U = 2.272 x 10^6 J * 1c/4.185 J = 5.429 * 10^5 c
Calorie
To increase the temperature of 1 gram of water one degree Celcius requires one calorie of energy the unit is named the specific heat with symbol, c, units J/kg °C or cal/g °C, for water c = 1 cal/g °C and c = 4184 J/kg °C
U = m c DT
To heat a kilogram of water from 0 °C to 100°C i.e. DT = 100 °C requires
U = mc DT = 1000 * 1 * 100 = 10^5 cal
or using SI units
U = mc DT = 1 * 4184 * 100 = 4.184 * 10^5 J
Note the food calorie, C, is 1000 thermal calories and is often capitalized 1C = 1000 c, needless to say this can lead to confusion!
The ideal gas law
The temperature, T, in kelvins, volume, V, in m^3, Pressure, P in pascals, and number of moles, n are related by the ideal gas law:
PV = nRT
where R is the ideal gas constant 8.31 Pa m^3/mol K
by memorizing this one equation you will see that when the number of moles is constant:
Pressure is proportional to temperature at constant volume.
Volume is proportional to temperature at constant pressure. Charles' Law
Put a flaccid balloon over the mouth of an empty 2 liter bottle and then run hot water over the bottle. Notice how the balloon inflates.
Volume is inversely proportional to pressure at constant temperature, Boyle's Law.
To demonstrate Boyle's Law, put 10 mL of gas into a syringe and cap the syringe. As you reduce the volume of the gas you can feel the increase in the pressure.
For pressure, a pascal is 1 N/m^2.
|
Scientific Explorations with Paul Doherty |
|
25 November 2005 |