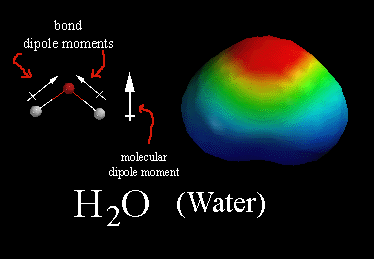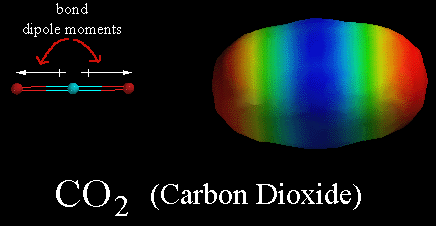Greenhouse Effect
The earth has a liveable temperature thanks to the greenhouse effect. Without greenhouse warming the average temperature of the earth would be -18 °C
with greenhouse warming it is closer to 59 °F or 15°C.
A teacher Institute workshop on 22 November 2008
Scale Models
To start consider the scale of the atmosphere of the earth.
Atmosphere Thickness Model Place a layer of plastic cut from a peanut butter jar, or from two layers of 2-Liter bottle plastic, onto an earth globe. 90% of the earth's atmosphere will be within this distance from the surface. Half the atmosphere is within 6 km of the surface which is 1/1000 the radius of the earth, 90% of the atmosphere is within 20 km or 1/300 the radius. With an earth globe of 15 cm radius this would be 15cm/300 or 1/20 cm or 1/2 mm or 0.5 mm.
Atmospheric Pressure bar, a 1 inch square 5 foot long steel bar weighs about 14.7 pounds, so the pressure it exerts is 14.7 pounds/in.^2, atmospheric pressure.

The gas that contributes the most to greenhouse warming is?
Water Vapor, H2O makes up a variable amount of the earths atmosphere, up to 4 percent in hot, humid conditions.
What is the Atmosphere made of? DRY air is 78% N2, 21% O2, and 1% Ar
However, real air has a variable amount of H2O up to 4%
It also contains trace gasses such as carbon dioxide, and methane.
Atmosphere Composition Model Model the composition of the atmosphere using dyed grains of rice.
 Stephanie Chasteen olds a 1 liter container with dyed rice grains showing the composition of the atmosphere.
Stephanie Chasteen olds a 1 liter container with dyed rice grains showing the composition of the atmosphere.
A second greenhouse gas is carbon dioxide, CO2, it makes up about 400 ppm, or 0.4 ppt or 0.04 % of the earth's atmosphere. So there is 100 times less CO2 than H2O in the atmosphere.
A third greenhouse gas is methane, CH4, it makes up 1.7 ppm of the earth's atmosphere. 0.00017 %
The lesson from other planets, thank a clam today.
Venus and Mars have atmospheres that are 95% CO2. Venus is so hot due to the greenhouse effect that lead would melt on the surface.
And Mars is so hot that on the surface at the equator in the summer water ice would melt.
At one time the earth's atmosphere might have been 95% CO2, where did it go? The CO2 dissolved in ocean water on the earth where it combined with calcium leached from rocks, organisms used calcium carbonate to make defensive shells. These shells were then buried making huge deposits of chalk and limestone essentialy turning the carbon dioxide into stone, calcium carbonate, and burying it. Take a moment to thank a clam today.
Here is a classic science question, I hand you a wooden log for a fireplace and ask where did the atoms this log is made of come from?
The carbon in the carbohydrates that make up the log came from carbon dioxide in the atmosphere. The bulk of the mass of the tree is made from a gas that makes up 0.04% of the atmosphere. When the tree dies, or any other plant, it may decay immediately releasing its carbon back into the atmosphere as carbon dioxide, or it may be buried and fossilized slowly turning into coal, oil or gas, all fossil fuels. This effectively removes CO2 from the atmosphere. Until the fossil fuel is removed from the ground and burned, returning to the atmosphere carbon dioxide that was removed during the carboniferous.
Carbon Dioxide
Carbon Dioxide Model You can model carbon dioxide using balls and hacksaw blades.

Carbon dioxide is a linear molecule with the carbon in the middle.
The molecule is electrically neutral, in the covalent bonds the oxygens are negative and the carbon is positive, carbon dioxide it is not a polar molecule. - ++ -
Compare this with the water molecule overall it is neutral, due to the covalent bonds the oxygen is negative and the hydrogens are positive, but water molecules are bent so the water molecule is a polar molecule with a positive part and a negative part.
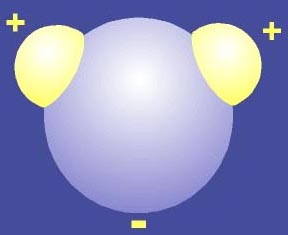 A water molecule the yellow ends are hydrogens while the blue is an oxygen.
A water molecule the yellow ends are hydrogens while the blue is an oxygen.
Electric Dipoles
In order to absorb or emit electromagnetic radiation well (according to the wave theory of 1870) a molecule must have a dipole moment that changes in time.
An electric dipole is made of two equal and opposite electric charges, +q and -q separated by a distance , d.
It has a magnitude called the electric dipole moment P = qd
It is a vector with a direction from the negative charge to the positive charge. It has units of coulomb meters.
When an electric dipole oscillates at a frequency, f, it gives off electromagnetic radiation at frequency f.
When electromagnetic radiation hits an electric dipole it causes it to oscillate.
Here is a web page about dipoles and chemical bonds http://www.chem.tamu.edu/organic/Spring99/dipolemoments.html
Water
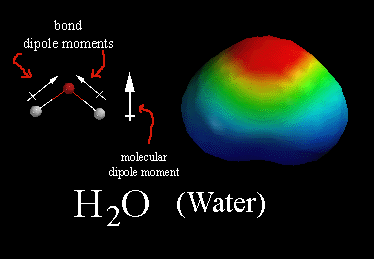
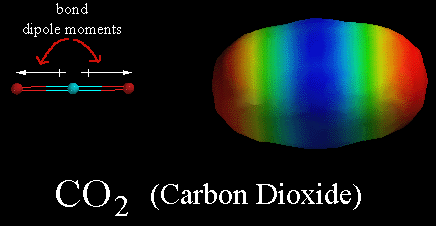
Red regions in these images are nagatively charged compared with blue regiins.
The CO2 molecule is not a dipole when it is at rest, however infrared radiations or collisions with other molecules can make it into a dipole.

If the CO2 bends then the negative oxygens move down while the positive carbon moves up creating a dipole moment, a little later the oxygens move up and the carbon moves down reversing the direction of the dipole, so we can have an oscillating dipole that absorbs and emits IR.
The size of an infrared wavelength compared to the size of a carbon dioxide molecule
The CO2 molecule is about 0.3 nm long, 3 x 10^-9 m
Infrared radiation absorbed by and emitted by CO2 has a wavelength of 15 micrometers, 1.5 x 1^ -5 m
So in one wavelength of infrared you could line up 5000 CO2 molecules.
And yet one CO2 molecule can absorb or emit one entire infrared photon. (Photon model of 1930)
A most important equation: frequency, wavelength and speed
f L = c The frequency of light times its wavelength equals the speed of light.
So if you know the wavelength of light you can always calculate the frequency.
The frequency of infrared light absorbed by CO2 is f = c/L = 3 x 10^8/ 1.5 x 10^-5 = 2 x 10^13 Hz
This is also the vibration frequency of the molecule.
There is another vibration of CO2 that creates an oscillating dipole it is modeled by this exploration with magnets, Carbon Dioxide Magnet Model.
In this oscillation the carbon dioxide remains a straight line but the carbon in the middle shuttles back and forth between the two ends of the molecule.
As it shuttles the two charges on one end get far apart increasing the dipole moment on that side, while the two charges on the other side get close together reducing the oppositely directed dipole moment. So the CO2 becomes an oscillating dipole, which can absorb and emit IR. This oscillation is at a higher frequency than the bending oscillation. It emits and absorbs IR with a wavelength of 4 micrometers, 4 x 10^-6 m, and has a frequency of 0.75 x 10^14 Hz or 7.5 x 10^13 Hz almost four times higher than the bending mode and yet still in the infrared part of the spectrum.
There is one more mode of oscillation of the CO2 which has the middle magnet fixed in place while the outer two magnets move inward together then outward together.
Follow the changing dipoles in this example and see why this mode of oscillation does not emit or absorb infrared.
gif animations of the vibrations of CO2 here http://science.widener.edu/svb/ftir/ir_co2.html
Going Further
Digital cameras can see infrared light, Look at the output from a TV remote control with a digital camera, the camera will detect the infrared light emited by the camera that your eyes cannot see.

Summary of the greenhouse effect from wikipedia
http://en.wikipedia.org/wiki/Greenhouse_effect
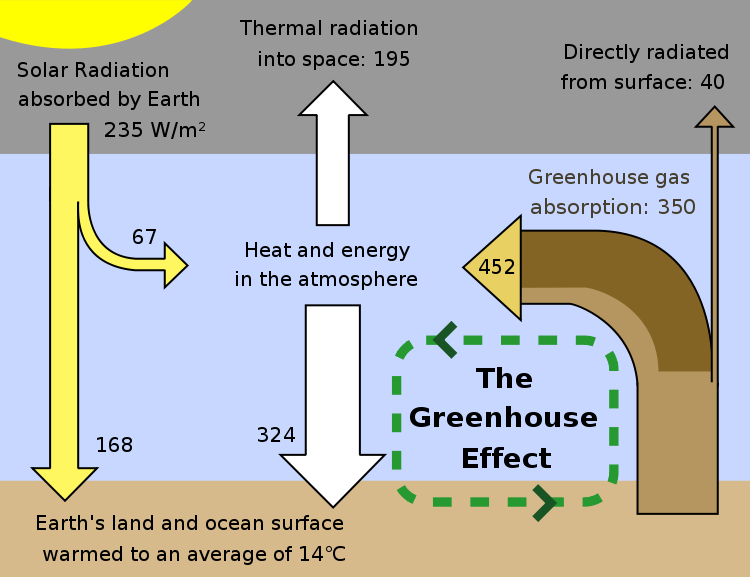
In equilibrium the amount of energy received from the sun must be balanced by the amount being radiated into space.
The only way energy can leave the earth and go into space is by radiation , convection and conduction will not work.
Even though the sun delivers 1000 W/m^2 it only does that during the day, so when we average over day and night this is reduced to 500 W/m^2, clouds, the atmosphere and the surface imediately reflect some of this radiation into space so that it is never absorbed. The end result is that on the average the earth absorbs 235 W/m^2 of sunlight.
If the earth had no atmophere the surface would heat up to an average temperature that radiated this energy directly into space, a temperature of -18 °C. the average temperature at the surface of the moon (which receives the same amount of sunlight per m^2 on average as the earth. )
The radiation from the sun which is nearly at 5700K has a peak in the visible, the radiation from the surface of an airless earth at 255 K is in the far infrared.
However, the earth has an atmosphere which contains gasses that absorb and reradiate far infrared radiation. When radiation is absorbed it is sometimes reradiated back down toward the surface. This warms the surface and is one model for the greenhouse effect.
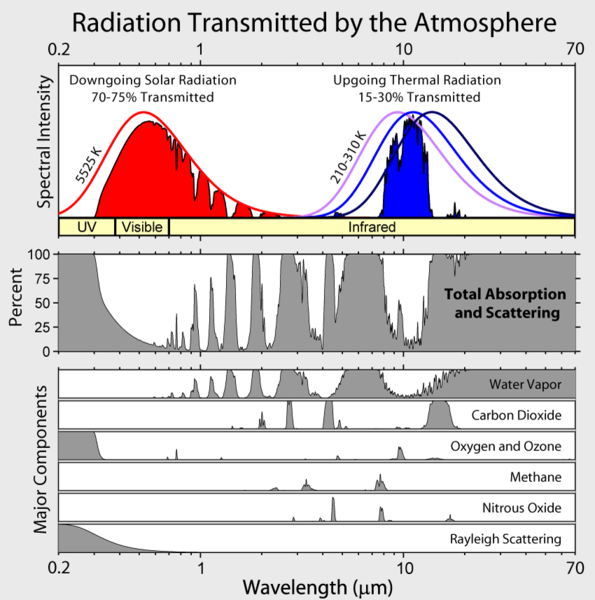
The above figure shows the details. Energy comes in in the visible is absorbed warming the surface and goes out in the infrared.
Note the amount of radiation absorbed by water vapor.
Note the aditional absorption due to carbon dioxide.
Note how ozone absorbs UVand a bit of IR, it is a greenhouse gas.
A Second Model for the Greenhouse Effect
We need to consider two important points before we get to the second Model
As altitude increases the temperature of the troposphere decreases by 6.5 °C per km of elevation. Why?
The temperature of the troposphere decreases with altitude because the surface is heated by incoming radiation, the surface then warms the air by conduction, the warm, expanded, less dense air then rises by convection. As the air rises the air pressure decreases and it expands. As it expands it does work reducing its internal energy and reducing its temperature, this is called adiabatic cooling. So as the air rises in the troposphere the temperature decreases, by the adiabatic lapse rate 6.5 °C per km.
Feel the air coming out of a bicycle tire and note that it is cool, the air has been cooled by adiabatic expansion.
Use the largest rubber band you can find, hold it against your lip and feel its temperature, stretch it hold it for a count to 10, hold it against your lips and let it return to its original length, notice that it becomes cool as it contracts. The rubber band does work as it contracts, the work comes from the internal energy cooling the rubber band.
Why does the sun have a visible Surface?
The density of the gas that makes the sun decreases exponentially and smoothly with altitude, there is not a trace of a discontinuity where we see the surface. This is a major difference compared to the earth where the density abruptly drops by a factor of 1000 at the surface.
A photon emitted by an atom or ion in the sun travels in a straight line until it is absorbed by another atom or ion. Deep in the sun where the atoms and ions are close together they emit a lot of photons, but these photons are certain to be absorbed before escaping from the sun. Higher up the atoms and ions become further apart so there is an increasing chance that the photon will be able to travel in a straight line and escape the sun, but there are fewer of them emitted. There is a level in the atmosphere where there are enough atoms and ions to emit a lot of photons and yet few enough that they escape. This is the level we see when we look at the sun.
What does this have to do with the greenhouse effect?
The same thing applies to infrared radiation emitted by the atmosphere of the earth, if you looked at the earth from outer space with eyes that saw far infrared radiation you would see a glowing opaque layer high up in the tropsophere.
Pit vipers like rattlesnakes see far infrared radiation with their pits so a snake in space would see this opaque layer.
There is a level in the atmosphere, in the troposphere where infrared radiation emitted by greenhouse gasses finally escapes to space. At equilibrium, this level has a temperature that emits enough radiation to balance the input of energy from the sun, Tg perhaps near -23 °C.
If we add greenhouse gasses to the atmosphere the density of carbon dioxide increases and the height in the atmosphere from which infrared is emitted to space becomes higher. The amount of CO2 in the atmosphere has increased by 25% over the last 150 years. The amount of CO2 in the atmosphere is expected to double in the next 100 years. If it doubles the surface temperature change would be + 1.2 °C which with feedback effects may reach 2.5 °C.
Ar equilibrium the temperature of this layer has to be high enough so that the energy radiated out equals the energy arriving from the sun. This is just about the same temperature as before. But initially, the higher elevation temperature is lower and so for a while the earth receives more incoming radiation than it emits as outgoing radiation, this causes the atmosphere to warm.
At the moment the absorption and re-emission of radiation is not in equilibrium, due to the increase in greenhouse gasses the earth is currently absorbing 0.85 W/m^2 more than it is radiating, this is causing the atmosphere to warm.
When equilibrium is re-established, the level in the atmosphere has nearly the same temperature as before, Tg.
Starting at the elevation of the layer in the atmosphere from which infrared radiation is emitted to space, the temperature increases as we go down toward the surface.
It increases by 6.5 °C per km. So if the altitude of this layer increases it is further down to the surface and the surface temperature increases.
Mountain climbers know that the temperature of the air decreases as you gain altitude.
Real Greenhouses
The Greenhouse Effect for greenhouses is not the Greenhouse Effect in the atmosphere.
Glass transmits near IR and is opaque in the far IR so you often hear that the greenhouse effect in a greenhouse is due to the fact that radiation from the sun can enter the greenhouse, be absorbed by the interior of the greenhouse and re-emitted as far infrared which cannot escpe though the greenhouse glass or plastic.
However, in 1909 R. W. Wood made two small greenhouses one with a glass roof which was opaque to infrared radiation and one with a roof made from plates of salt which is transparent to infrared radiation. Both greenhouses when placed in the sun reached the same temperature of 55°C. This shows that the blocking of infrared radiation in the greenhouse plays a small part in incresing the temperature of the greenhouse, what the greenhouse really does is to block the convection of the gas. The gas warmed in the greenhouse remains in the greenhouse.
This same result applies to automobiles as well.
Conduction, Convection and Radiation, an investigation using thermochromic liquid crystals.

Return
to Draft Activities

 Stephanie Chasteen olds a 1 liter container with dyed rice grains showing the composition of the atmosphere.
Stephanie Chasteen olds a 1 liter container with dyed rice grains showing the composition of the atmosphere.
 A water molecule the yellow ends are hydrogens while the blue is an oxygen.
A water molecule the yellow ends are hydrogens while the blue is an oxygen.