Electrostatics
Flying Tinsel An electrically charged metal pieplate can be used to fly a ring of metal tinsel with the same charge.
Electrostatics workshop: Includes Charge and Carry, and Electroscope
Electrostatic and magnetic forces cross a vacuum.
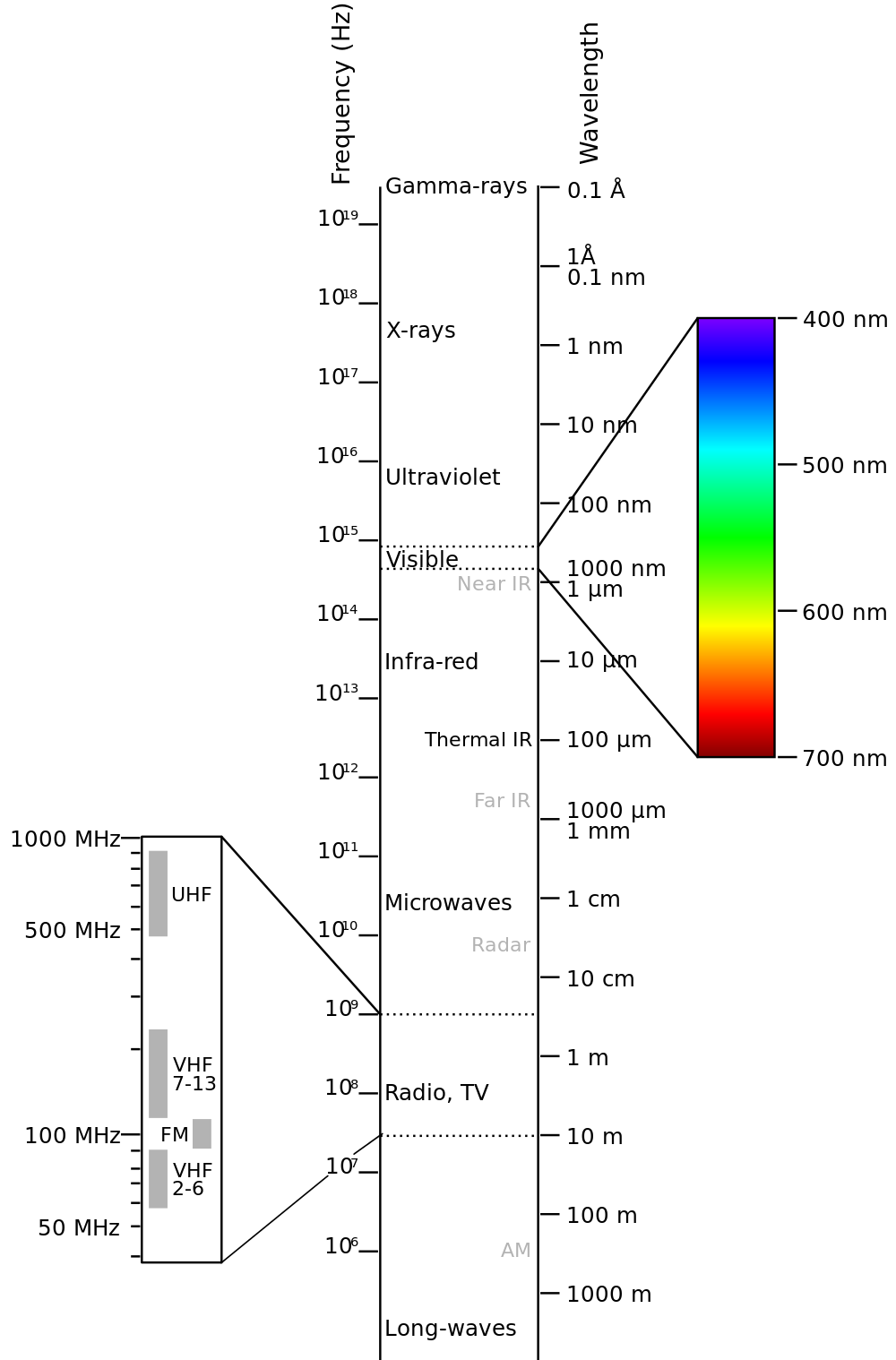
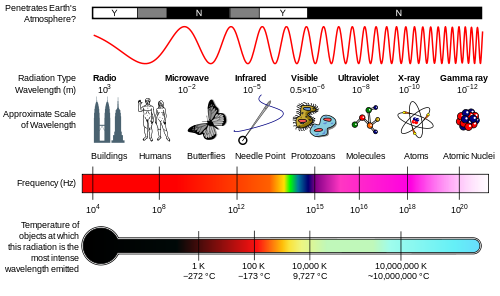
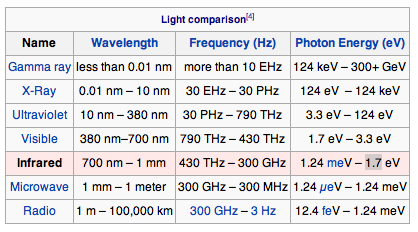
Electromagnetic Spectrum
Wikipedia Summary http://en.wikipedia.org/wiki/Electromagnetic_spectrum
What is Light? Historical survey with experiments
Spectra Explorations Use a diffraction grating
Color Exhibits, classroom explorations,and diagrams
Energy Level model using the center of mass of a chair
Solar brightness Measure the energy output of the sun in visible light using a grease spot photometer.
Infrared remote control Digital cameras can see into the near infrared.
Solar Thermal Measure the total energy output of the sun including infrared.
Heat Camera Like snakes this camera sees in the far infrared
The suns energy 1 KW/m^2: 527 W IR, 445 W Vis, 32W UV
Visible light red is 700 nm to violet 380 nm wavelength
Infrared : Discovered by William Herscel in 1800, 700 nm to 1 mm, 430 THz to 300 GHz, Thermal IR from humans peaks at 10 μm
Terahertz or sub-millimeter radiation used in airport scanners, it penetrates clothing and a little skin. This radiation was recently added to the spectrum between infrared and microwave.
Microwaves are used in radar and in microwave ovens and to transmit wifi.
The big bang is imaged in long wavelength microwaves.
Radio waves AM, FM. You can transmit to an AM radio by rubbing stranded wire between the terminals of a 9 volt battery to make sparks.
Ultraviolet was discovered by Johann Ritter 1801. It darkened silver salts.
Ultraviolet Comes in 3 "colors"
UV-A which is closest to violet light penetrates skin, can cause tanning and sunburn,
UV-B which is much more effective at causing sunburn and breaks bonds in DNA
UV-C germicidal but has a very short range in air
x-rays are emitted by the inner electrons of atoms and can ionize air. Discovered by Wilhelm Roentgen 1895.
gamma rays are emitted by atomic nuclei: Discovered by Paul Villard in 1900
Electromagnetic spectrum
sinusoidal waves of electric fields at right angles to magnetic fields each creating the other which can travel through empty space at the speed of light in a vacuum
c = 3 x 10^8 m/s
They have wavelength L, the distance between adjacent wave crests, measured in meters, m.
Period P the time it takes one complete wavelength to pass a point, measured in seconds,s.
The frequency, f, the number of wave crests that pass each second, measured in hertz, Hz.
f = 1/P
c = f L
Einstein discovered that electromagnetic waves come in quanta later named photons.
The energy of a photon E, in joules, J, is proportional to its frequency E = hf where h is Planck's constant = 6.6 x 10^-34 Js
So every blue photon with twice the frequency of a red photon has twice the energy of the red photon, but a source might emit more red energy simply by emitting twice as many red photons as blue ones.
Because each UV, x-ray and gamma ray photon has enough energy to break molecular bonds they can break DNA apart and cause mutations. Spectral components with frequencies of visible light and below cannot directly cause mutations.
Because electric and magnetic forces can cross a vacuum, electromagnetic waves can also cross a vacuum.


Web Resources
LIGO: http://www.ligo.caltech.edu/
LIGO Livingston Science Education Center: http://www.ligo-la.caltech.edu/worksheets/SEC/sechome.html
The Exploratorium: http://www.exploratorium.edu/
Teacher Institute: http://www.exploratorium.edu/ti/
Index of activities: http://www.exploratorium.edu/educate/index.html
Iron Science Teacher: http://www.exploratorium.edu/iron_science/index.php
Images and videos for teachers: http://nsdl.exploratorium.edu/nsdl/welcome.do
Exploratorium You Tube: http://www.youtube.com/user/Exploratorium
Paul Doherty: http://www.exo.net/~pauld/
List of activities: http://www.exo.net/~pauld/site_map.html
Lori Lambertson: http://philo.exploratorium.edu/~loril/
Eric Muller: http://www.exo.net/%7Eemuller/
Larry Braile Earth Science: http://web.ics.purdue.edu/~braile/indexlinks/educ.htm
|
Scientific Explorations with Paul Doherty |
|
26 Oct 2013 |